TIMS Laboratory
In our TIMS lab, we determine radiogenic isotope ratios of Os, Sr and Nd. For cosmochemical applications, the variations of stable and radiogenic isotopes of Cr are also analyzed.
Thermal ionization mass spectrometry (TIMS) at FU Berlin
Building B, Room B214
Lab Manager PD Dr. J. Elis Hoffmann
Thermal ionization mass spectrometry (TIMS) is an analytical method for high-precision measurements of isotope ratios and isotope abundances of elements that are easy to ionize. In geology, the method is mostly applied to measure the isotope abundances of radiogenic isotopes (which in nature often strongly varies) in relation to stable isotopes of the same element. Radiogenic isotopes are the decay products of long-lived radioactive isotopes (e.g., 87Rb, 147Sm or 238U). We apply this method to constrain ages of rocks and to use radiogenic isotopes as a tracer for mass transport during surface and subsurface processes.
We measure isotope ratios of elements with low ionization energy as positive ions (e.g., Sr, Nd, Pb). Some isotope ratios of elements that are not easy to ionize can also be measured using TIMS if boundary conditions are modified. Here, the many elements can be measured as negatively charged ions, e.g., BO2-, OsO3-, ReO4-, Se-, Te-.
In our TIMS lab, we measure radiogenic isotopes of Sr, Nd, and Pb. For cosmochemical applications, the variations of stable and radiogenic isotopes of Cr and Ba are also analyzed.
Moreover, in combination with the isotope dilution method we also use TIMS to determine the precise concentrations of various elements. At FU Berlin, we determine Rb, Sr, Sm, Nd, U, Pb, and Os concentrations for radiometric dating using TIMS.
If you have any questions concerning TIMS methods and applications, please contact
PD Dr. J. Elis Hoffmann and Prof. Harry Becker
During the winter term, we offer a 2-week lab course on geochronology. This is a hands-on introduction in geochemical methods on how to do element separation in a clean lab environment and how to measure isotope ratios using TIMS (see FU Berlin course catalog, J. Elis Hoffmann). This course serves M.Sc., B.Sc. and Ph.D. students who focus in their studies on geochemistry topics.

|
Thermo Electron Triton Multicollector-TIMS (set up in 2005) |

|
Heating device for filament cleaning (by K. Hammerschmidt, 2005) Background: packed magnet of the Triton instrumentation |

|
Heating device without protection (view from top) |

|
Device for welting PT filaments of filament holder |

|
Loading box for Re and Ta filaments (Nd, Sr, Pb) |

|
Loading box for Pt filaments (Os) |
TIMS measurements
The solution of an element that has been chemically separated from other elements is loaded (usually in a dilute acid) onto a high-purity metal ribbon (filament) made of Re, Pt or Ta and is dried up. The ion source of the TIMS heats the filament up to temperatures of up to 2000 °C (depending on the element) using a current under high vacuum. Thus, salt evaporates and atoms ionize. In a vacuum ions are accelerated using high voltage (mostly 10 kV) while the ion beam is brought into a rectangular shape with various apertures and focused in a gap. A strong magnetic field deflects the ion beam into a circular path. Light ions are deflected stronger than heavy ions. At the same time, the ion beam is focused so that all ions of the same mass are bundled in an exit slit. Mass dispersion enables simultaneous registration (multicollection) of the ion current of different isotope masses arranged in parallel in Faraday cages (cups). With the Triton instrumentation, it is possible to measure the ion currents of a maximum of 9 isotopes at the same time. The current in the cups (typically about 10-12 to 10-10 A) generates a voltage via high-ohm resistors and amplifiers that is recorded. Results for isotope ratios are calculated based on the ratios of the currents and voltages, respectively, measured in the various cups. In case of very low ion currents (<10-¹³ A), measurements are carried out with a secondary electron multiplier (SEV). Here, a change in the strength of a magnetic field requires jumping between different mass peaks (peak hopping).
Precision of measurements
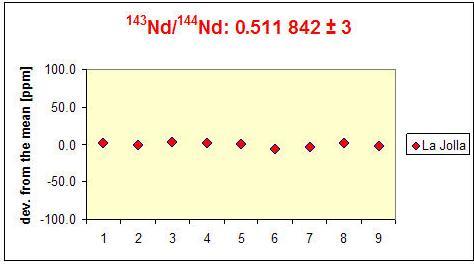
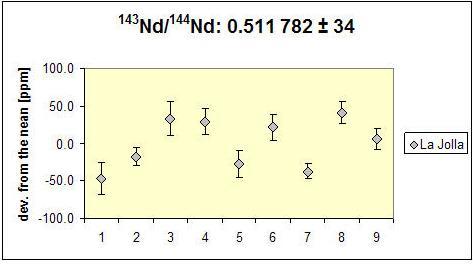
The precision of measurements for the TIMS instrumentation depends on a number of factors. In particular, important for an accurate measurement are the purity of the element to be measured, the intensity, and stability of the signal of the isotopes, the stability of the amplifier and that of other electronic components as well as the quality of the cups. The figure below compares the reproducibility of single measurements of the 143Nd / 144Nd ratio using the MAT 261 TIMS (disposal 2011, below) and the current Triton TIMS (acquisition 2005, above). The differences reflect both the age difference between the two instruments and a number of improvements concerning the Triton. The error bars of the individual measurements (2σm) are indicated, if not, they are smaller than the size of the symbols. In the long term, the Triton achieves an external reproducibility of the 143Nd / 144Nd ratio in the order of a magnitude of ± 10 ppm (2σ) and better since the error bar scatter decreases from measurement to measurement.
|
Triton |
|
MAT 261 |