Thermodynamics of fluids and rocks
P/T Phase diagram of a hydrated peridotite, calculated with THERMOLAB
Image Credit: Plümper et al., 2017
Understanding the thermodynamic behavior of fluids and rocks is crucial to correctly interpret mineralogical and petrological observations in the field. Thermodynamics is used in chemical equilibrium calculations for phase diagrams, as well as in numerical modelling of non-equilibrium processes such as reactive fluid flow and rock deformation. As fluids facilitate reactions in rocks which in turn affect transiently its flow -sensitive porosity, it is of main importance to properly characterize the thermodynamic behavior of geological fluids. We focus on quantification of the composition of fluids and minerals using theory, experiment, and observation.
Behavior of fluorine, chlorine and the other halogens in aqueous solutions
Mobilization and transport of elements in the Earth's crust are important processes for the formation of ore deposits and for global element cycles.
The primary transport mechanisms involve either melts or fluids as mobile phases. Anions play a major role in controlling the transport of cations in fluids by complexation. Most economically interesting elements, including the so called "High Tech Metals" such as lanthanides (Rare Earth Elements), are transported mainly as cations complexed by anions in fluids. Chlorine and fluorine are the two most abundant halogen elements on Earth and two of the most important anions in geological fluids. Understanding the influence that these elements have on cation transport is therefore crucial. Combining observations on natural systems from the large scale of entire ore deposits to the small scale of high-resolution electron microscopy with numerical computer modelling provides the most detailed insights into such problems. However, the computer models require input data in form of the physical and chemical properties of the elements of interest under the high pressures and temperatures in the Earth's crust. While some such data are available, tackling new and exciting questions often means that we need to create the relevant data ourselves by laboratory experiments. Especially the complexation of metals critical for high tech applications with fluorine is not yet well known and one of the focus points of our group.
Further reading: Manzini et al. (2017); Wudarska et al. (2021)
Composition and chemical effects of fluids
Although it is very well established that fluids play a major role in almost all geological processes and control key parameters in geodynamics, the fact that most of the time fluids have left the rock after the interaction process makes it difficult to decipher the composition of fluids at depth. We are mostly left with the solid rock product that bears witness of fluid-rock interaction processes and we have to use this to interpret the composition of fluids involved in the process. Analyzing the mineral chemistry and isotope geochemistry of carefully selected samples, it is possible to constrain possible fluid compositions that controlled key processes in the rocks. In combination with partition coefficients and fractionation factors, this information can be applied to constrain models on how fluids flow through geological systems, such as subduction zones. By designing numerical models that can simulate the observed processes, we have another tool to further constrain what fluid composition was responsible for the mineralogy, structures, and geochemistry of the rocks.
Further reading: Chen et al. (2019); Li et al. (2020); König et al. (2020)
Thermodynamic modelling
We use Thermolab (Vrijmoed & Podladchikov 2022) for thermodynamic modelling to compute phase equilibria and to do transport modelling. The software is based on calculating Gibbs energies for solid solutions, charged and neutral aqueous species, molecular fluid models and melts combined with Gibbs minimization or using equilibrium constants. Written in native MATLAB, the chemical equilibrium solver is combined with numerical models of material transport, heat flow, and deformation. As it is being developed in the Mineralogy-Petrology working group, it continues to be updated, benchmarked, and calibrated to expand the range of applications. Examples of the usage of the software can be seen for example in the project of modelling dehydration in subduction zones, the modelling of soapstone reaction fronts, or the development of metasomatic zoning.
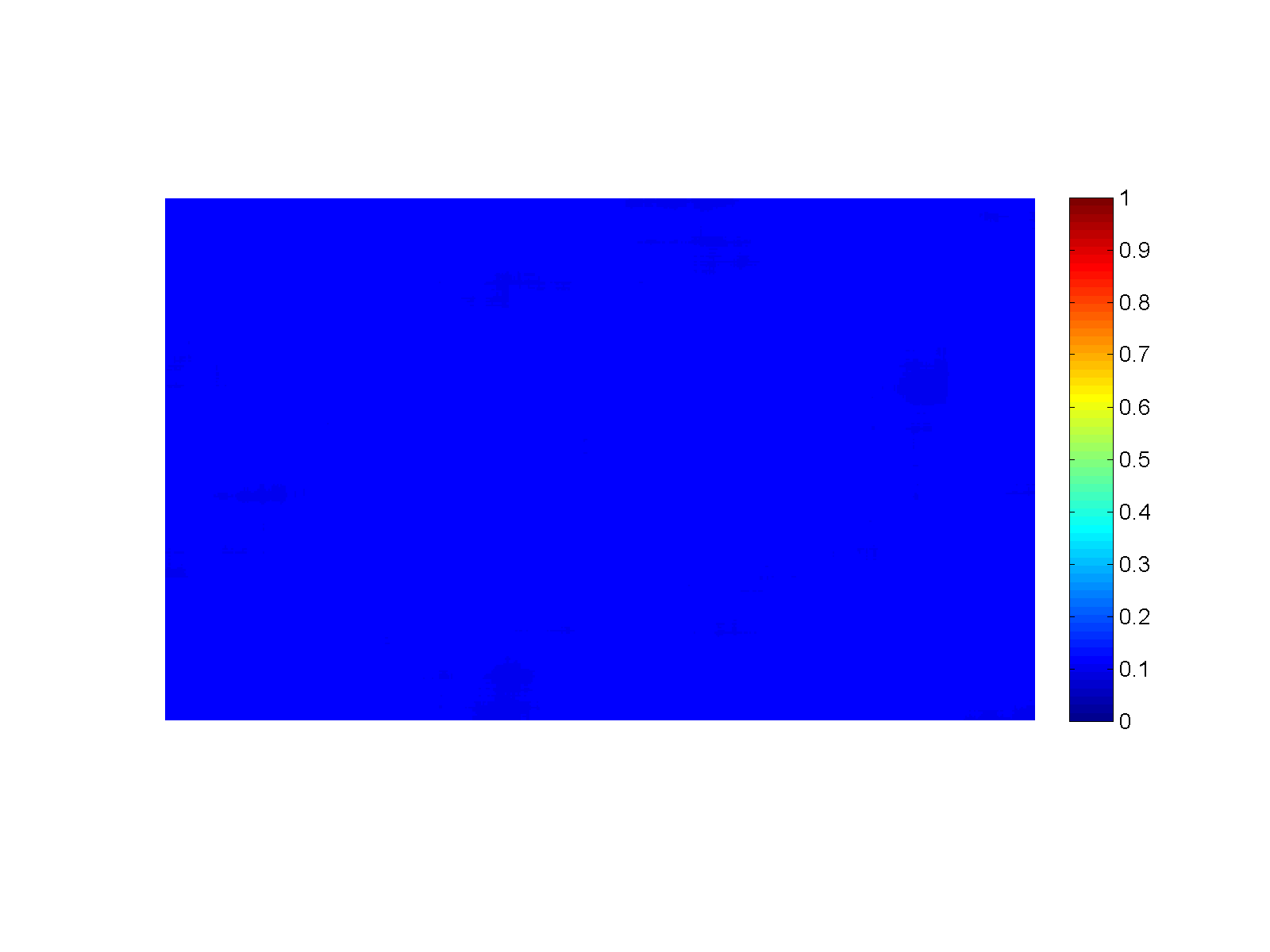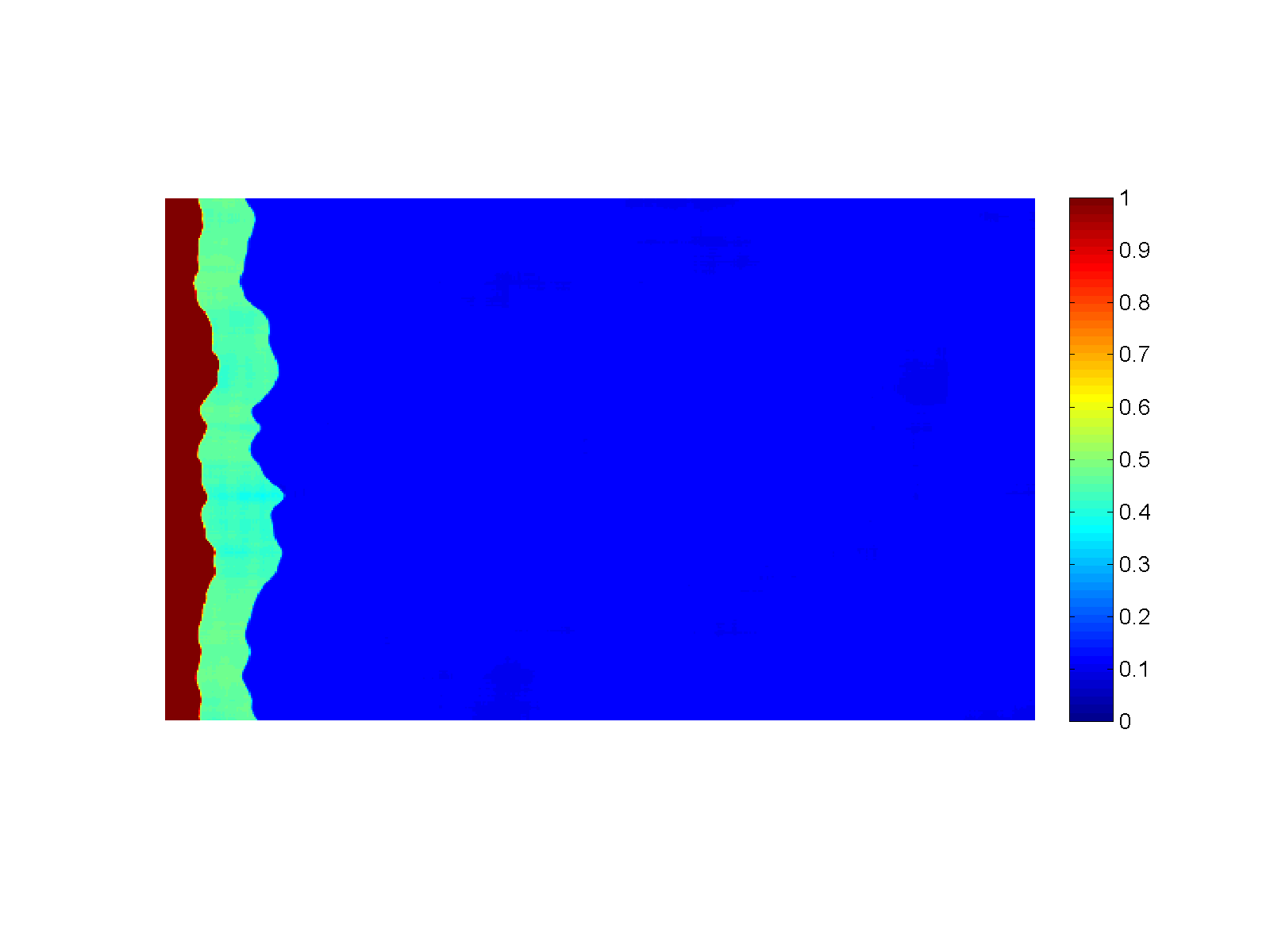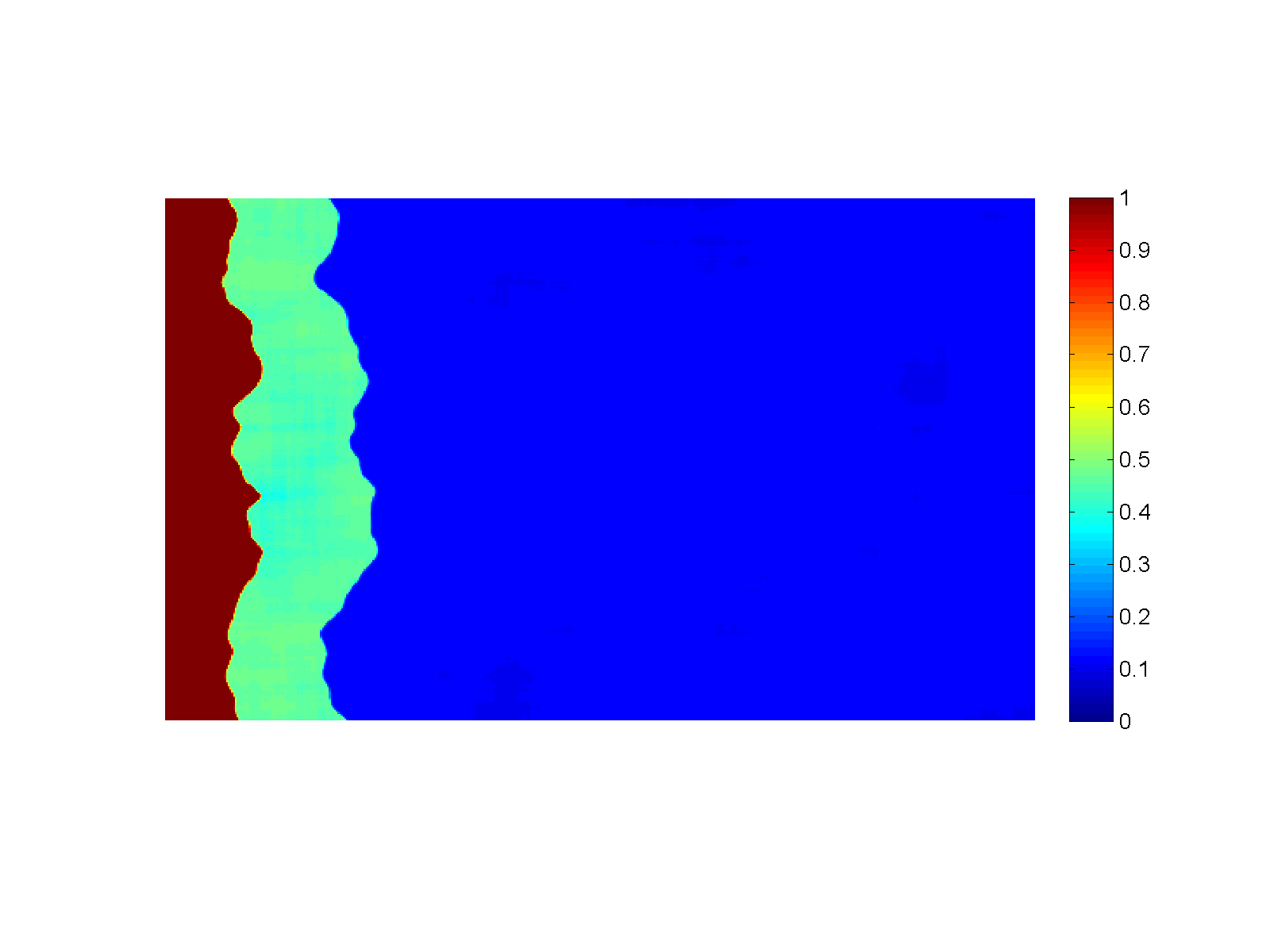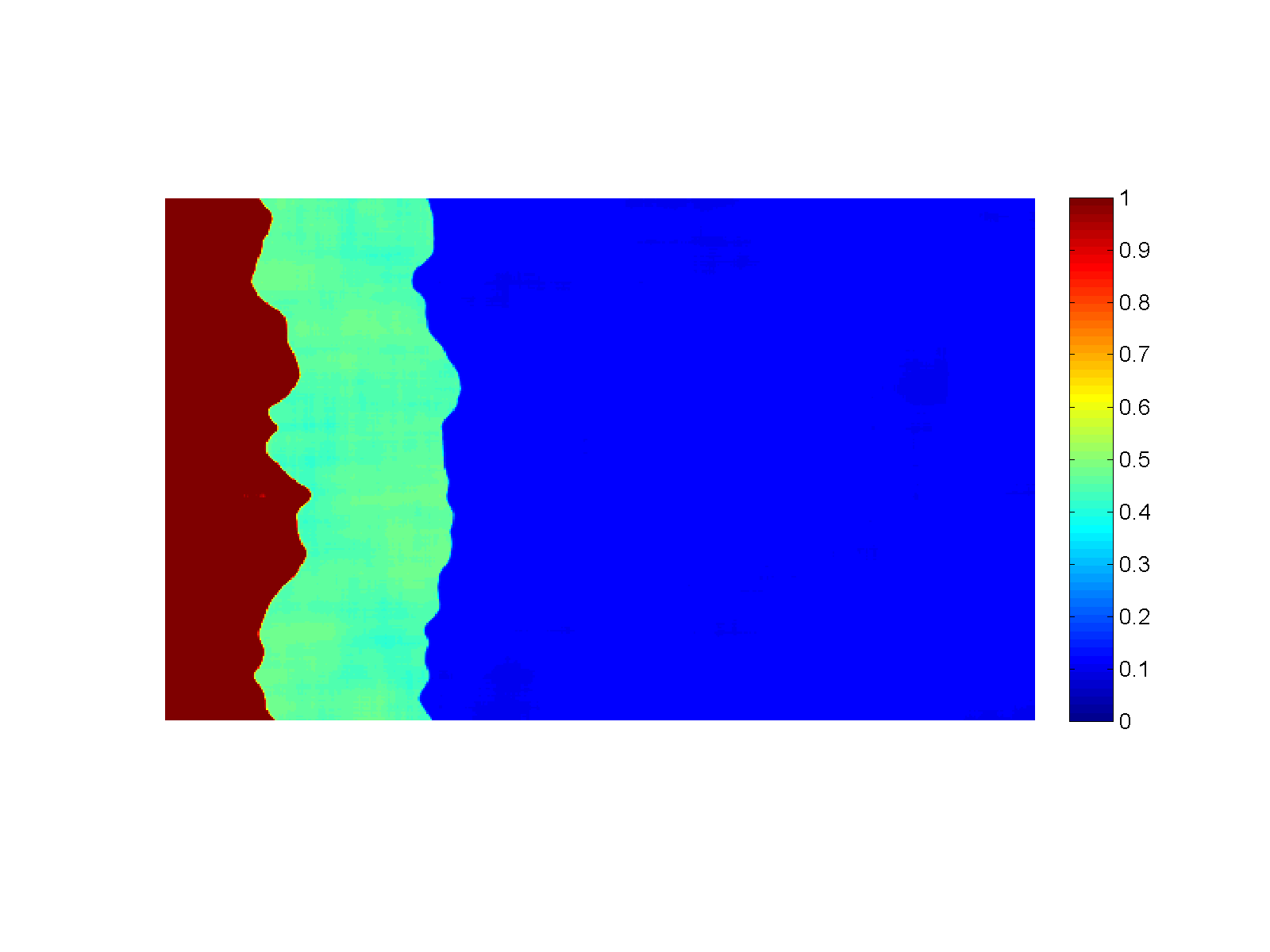
Figure (Simulation): Generation of sharp reaction fronts in peridotite resulting from reactive fluid flow. Fluid enters the domain in a thin fracture on the left and infiltrates to the right into the wall rock. A monomineralic garnet vein (red) is formed on the left, preceded by a pyroxenite layer (green). Click on the figure for a larger view!
Further reading: Plümper et al. (2017); Beinlich et al. (2020); Vrijmoed & Podladchikov (2022)